-
Products
Inspection Solutions Overview CapWatcher Q-Line CapWatcher B-Line CapWatcher SC CapWatcher FC Closure periphery IntraOne BarrierWatcher
Are you Tethered Caps ready? We are.
Find out about the vision inspection of tethered caps with our CapWatcher Q-Line.
How can Artificial Intelligence and Deep Learning be used in vision inspection? In which applications is it beneficial?
- Industries
- Service
- News & Blog
- About us
- Career
- Contact
Products
Preforms
Industries
Service
News & Blog
About us
Career
Contact
Inspection Solutions Overview
PreMon
PreWatcher Offline
PreWatcher Inline
Sample-PreWatcher
LayerWatcher
ColorWatcher Lab
Caps & Closures
Inspection Solutions Overview
CapWatcher Q-Line
CapWatcher B-Line
CapWatcher SC
CapWatcher FC
Closure periphery
IntraOne
BarrierWatcher
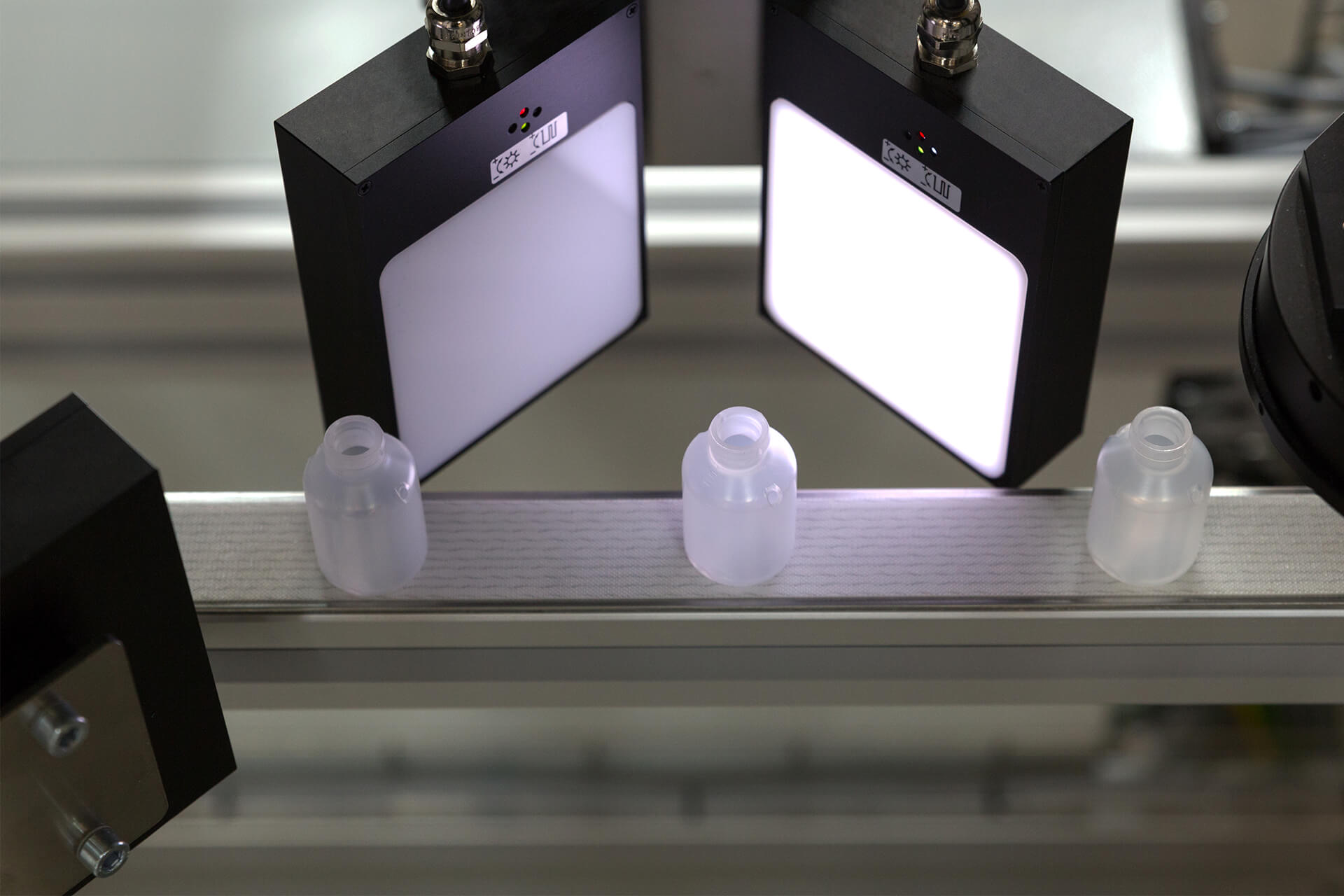
Bottles & Containers
Inspection Solutions Overview
SpotWatcher Customized
SpotWatcher Basic
SpotWatcher Pharma
BarrierWatcher
IntraOne
Decoration & Labeling
Barrier Layer
Software